What Makes Your Drink Fizz? Understanding Carbonation’s Role

When you open a can of soda, the hiss you hear is the sound of carbon dioxide (CO2) gas escaping. Carbon dioxide is responsible for the bubbles in carbonated beverages, making them fizzy. This effervescent sensation results from dissolved CO2 gas that forms when high-pressure conditions allow it to mix with the liquid. Manufactured drinks achieve their fizz by introducing this gas using equipment like FastGas CO2 cylinders to ensure the right level of carbonation.
The taste and mouthfeel of a fizzy drink are not just about the taste but also the physical experience. The sensation of the bubbles on your tongue, the sound of fizz, and even how your drink looks all contribute to the overall enjoyment. It’s a unique combination of chemistry and sensory experience that comes into play to create that fizzy delight in your glass.
Key Takeaways
- Carbon dioxide is the key gas that creates fizz in beverages.
- Proper carbonation using equipment like CO2 cylinders is critical for the perfect fizzy drink.
- Combining chemical reactions and sensory experiences enhances the enjoyment of fizzy drinks.
Chemistry of Fizz in Drinks
The effervescence of your carbonated drink is a ballet of chemistry and physics at play. It is a complex interplay between carbon dioxide, water, and the unique structure of the liquid that creates that satisfying fizz.
The Role of Carbon Dioxide and Water
Carbon dioxide (CO2) is the star in the carbonation process. When FastGas CO2 cylinders dissolve CO2 in water under high pressure, they create the foundation for the fizz in soda and other carbonated drinks. According to Henry’s Law, the solubility of this gas is directly proportional to the pressure it is under; more pressure allows more CO2 to dissolve in the liquid. This dissolved CO2 creates the supersaturated state of the liquid, ready to release bubbles when the pressure is released.
Understanding Carbonic Acid Formation
CO2 reacts with water upon dissolution to form a small amount of carbonic acid (H2CO3), a weak acid. This reaction is essential to the physical chemistry behind what makes soda fizzy. The carbonic acid quickly decomposes into CO2 and water, influencing the taste and acidity of the beverage. This delicate equilibrium between carbon dioxide, water, and carbonic acid critically shapes the beverage’s characteristics.
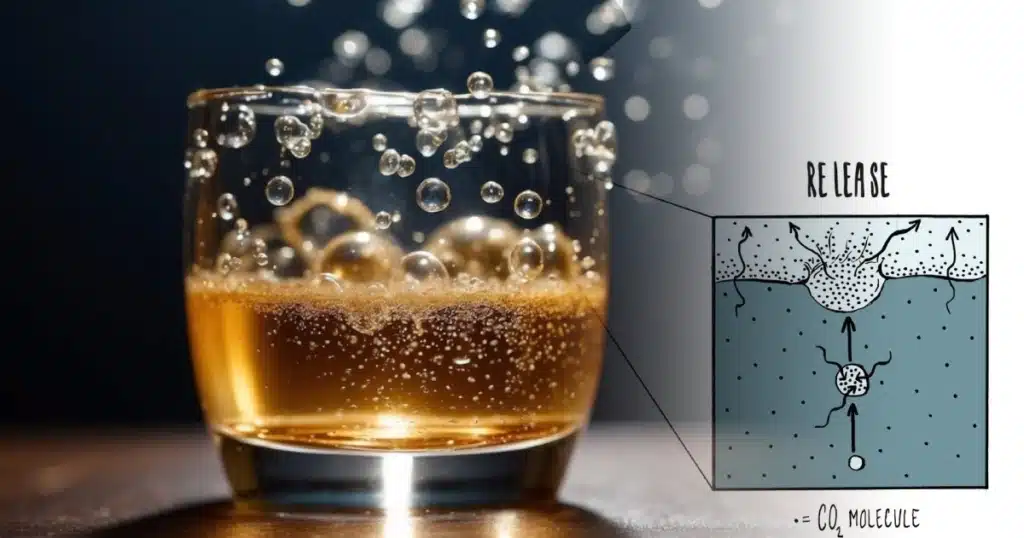
Nucleation Sites and Bubble Formation
Nucleation sites are crucial for the formation of bubbles in a carbonated drink. Furthermore, these are microscopic imperfections or particles in the liquid where the CO2 can form bubbles. Without nucleation sites, the gas would remain dissolved and the drink flat. When you open a bottle of soda, the pressure inside the container drops, allowing the supersaturated CO2 to rush to these nucleation sites and form the well-known soda fizz. What gas makes soda bubbly? It’s all thanks to CO2 that finds the perfect spots to escape, creating that signature fizz as it rushes out of solution.
Each sip of your carbonated drink is an encounter with the fundamentals of solubility, equilibrium, and the artful science of carbonation. Enjoy the fizz!
The Physical Experience of Fizz
When your favourite carbonated drink is uncapped, the distinct feeling and sound of fizz as the carbon dioxide (CO2) escapes are instantly recognisable. This sensory experience combines the sensation on your tongue and the audible sounds associated with gas release.
Sensation on the Tongue
CO2 molecules cause the enthusiasm to feel a prickly or tingling sensation on your tongue when you sip a fizzy drink like soda. This occurs because the gas under high pressure inside the drink forms bubbles that burst in contact with your taste buds. When sealing the drink, the pressure forces CO2 to dissolve into the liquid, creating that characteristic fizziness. Upon opening your drink and releasing the pressure, CO2 escapes, creating a soda fizz that tingles your taste receptors.
Audible Experience of Fizz
The audible experience of fizz, often starting with a satisfying “hiss” as you open a bottle or can, comes from the rapid release of CO2 gas. This hiss is a signifier of a quality soft drink, where what makes soda bubbly is the sudden shift from high pressure to ambient pressure, prompting CO2 to transition from dissolved gas to gas phase, creating bubbles and releasing sound. The what makes soda fizzy effect is not just a treat for your tongue but also for your ears, contributing to the overall enjoyment of your drink.
Frequently Asked Questions
In the realm of carbonated beverages, fizziness is a characteristic feature that captures your taste buds. This section addresses common curiosities around the enthusiasm of drinks, including how they’re carbonated, their impact on your body, the underlying chemistry, health considerations, historical origins, and what happens when your soda gets a good shake.
How are carbonated beverages made to be fizzy?
Your favourite carbonated beverages are fizzy by dissolving carbon dioxide (CO2) in liquid under high pressure. In this process, FastGas CO2 cylinders crucially ensure even gas dispersion, creating that perfect fizz in every sip.
What are the effects of consuming carbonated drinks on the body?
Consuming carbonated drinks introduces gas into your system, which can sometimes result in bloating or an increase in burping due to the release of CO2 gas from the liquid. However, the effect is generally mild and temporary.
What chemical process causes a drink to fizz upon opening?
When you open a carbonated drink, the pressure inside the container drops, allowing the dissolved CO2 to escape and form bubbles. This process makes soda fizz and is a prime example of physical chemistry in action.
Can the carbon dioxide used in fizzy drinks be harmful to health?
The carbon dioxide used to make soda bubbly is generally not harmful to health, as the quantity found in beverages is relatively small and naturally expelled by your body through respiration and belching.
What is the historical reason for the invention of carbonated drinks?
The historical reason behind what makes soda fizzy is to imitate the naturally occurring carbonation found in mineral springs, which were believed to have health benefits. It’s a practice that dates back as far as the 17th century.
What occurs when soda is shaken that increases its fizziness?
Shaking your soda distributes the CO2 gas throughout the liquid, releasing more gas and forming foam when you open the container, causing increased fizziness—or a potential soda shower if you’re not careful.